Hard Water: The Science behind This
Hard water is water that has a high mineral content. This content usually consists of high levels of metal ions, mainly calcium (Ca) and magnesium (Mg) in the form of carbonates, but may include several other metals as well as bicarbonates and sulfates.
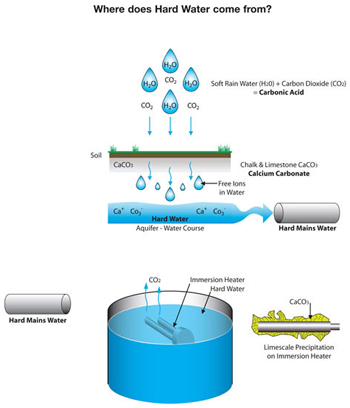
How the hard water is form? The main reason is , the rain becomes acidic is because Carbon Dioxide gas (the greenhouse gas) becomes absorbed by the rain turning it into a weak acid called Carbonic acid.
H2O+CO2=H2CO3
water + carbon dioxide = carbonic acid
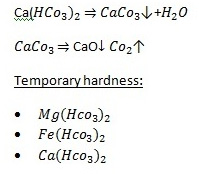
Hardness is caused by compounds of calcium, magnesium, and a variety of other metals. All freshwater sources contain calcium and magnesium in varying quantities. Water dissolves, suspends, and/or exchanges certain trace elements and compounds from many things.
Total water hardness (including both Ca and Mg) is reported as ppm w/v (or mg/L) of CaCO3. Water hardness usually measures the total concentration of Ca and Mg, the two most prevalent divalent metal ions, although in some geographical locations iron, aluminium, and manganese may also be present at elevated levels. Calcium usually enters the water from either CaCO3, as limestone or from mineral deposits of CaSO4. The predominate source of magnesium is dalmatic limestone, CaMg(CO3)
| Carbonate hardness compounds | Noncarbonate hardness compounds |
| Calcium carbonate (CaCO3) | Calcium sulfate (CaSO4) |
| Magnesium carbonate (MgCO3) | Magnesium sulfate (MgSO4) |
| Calcium bicarbonate (Ca(HCO3)2) | Calcium chloride (CaCl2) |
| Calcium bicarbonate (Ca(HCO3)2) | Magnesium chloride (MgCl2) |
| Magnesium bicarbonate (Mg(HCO3)2) | Carbonate hardness compounds |
| Calcium hydroxide (Ca(OH)2) | Sodium Fluoride (Na3AlF3) |
| Magnesium hydroxide (Mg(OH)2) | Calcium Fluoride (CaF2) |
Gridding of water hardness
|
 |
